Tuesday, April 3, 2012
Glowy!
Have you ever seen a black light? Most people have, but probably don't know what it is. A black light is much different than your typical "normal" or white light. The human eye sees the range of the colors of the rainbow. Nothing more. White light is the light the sun gives off. Man-made white light is the brightest light bulb you've ever seen. The white light has the colors of the rainbow in it, the most often seen being red, orange, yellow, green, blue, indigo, violet. Now black light, or essentially no light at all, doesn't have any of these colors. (Now you're probably looking up into space thinking, "There's no light up there, no colors, those aliens must not need to see!" but there is a wider range of light and color, in which cannot see. We probably see as much to the aliens as a black and white movie.) If you look at the keyboard in front of you, you see, whatever color the keyboard is. That is the color that white light reflects to your eyes, so it's essentially every color but that. Take a highlighter, and draw somewhere on your body. It doesn't matter where, but keep it appropiate. You can barely see it, right? That's because white light does not reflect much off of highlighter ink. (You may think it reflects well on paper, but just wait) Now highlight on a piece of paper. It's very bright, yes? Now take a black light and shine it on the highlighter on the paper and your skin. On your skin, you can see exactly what you wrote, quite clearly, correct? And on paper, it's very bright. This is because the highlighter reflects the darker light rays better than the white light rays. If you find a scorpion, shine the black light on it, but with caution. You'll notice it glows. This is because it's blood contains substances that reflect dark rays much better than white rays. Have fun coloring yourself with highlighter!
Monday, April 2, 2012
Electricity
Electricity. What a mysterious thing. It seems as though when you can't see something, you have no idea what it is. Which only powers the human's gift of curiosity. Electricity is the flow of charge from one point to another, usually - to +. There MUST be a complete circuit for the electricity to flow from point A to point B. No questions asked. Do you ever wonder what particle is required for electricity to flow? I give you one guess. That's right! The electron. (If you couldn't guess that, you may need help, being that the two words alone start with electr.)
Wednesday, March 28, 2012
Chemical Nomenclature
Chemical Nomenclature is the term given to the naming of compounds. Let's focus primarily on the forming of ionic compounds. Generally, and ionic compound forms when a metal and a non-metal are combined.
Thursday, March 15, 2012
Liquid Nigrogen
Liquid nitrogen is nothing short of its name; the liquid state of the element nitrogen. The coumpound can be found in the atmosphere under temperatures of 63 K and 77.2 K (-346 F and 320.44 F). Below 63 K, nitrogen freezes into a solid; under 77.2 K, nitrogen becomes a gas.
It is normally obtained from the attmosphere thus making it inexpensive and therefore is rarely refrigerated. Liquid nitrogen is stored in containers known as Dewars and is left to boil away. Since it boils, Laboratories have liquid nitrogen that has been shown to have temperatures of 77.2 K. Liquid nitrogen looks like boiling water, but it is extremely cold; it can be very dangerous to a human when in direct contact but it can be very usefull in science.
Reference: http://education.jlab.org/qa/liquidnitrogen_01.html
It is normally obtained from the attmosphere thus making it inexpensive and therefore is rarely refrigerated. Liquid nitrogen is stored in containers known as Dewars and is left to boil away. Since it boils, Laboratories have liquid nitrogen that has been shown to have temperatures of 77.2 K. Liquid nitrogen looks like boiling water, but it is extremely cold; it can be very dangerous to a human when in direct contact but it can be very usefull in science.
Reference: http://education.jlab.org/qa/liquidnitrogen_01.html
Tuesday, March 13, 2012
How an Engine Works
· Different types of engines
o Diesel
o Gas turbine engines
o Internal combustion engines
§ Gas is burned on the inside
§ The main idea/theory
· If you put a high power fuel (gasoline) in a small confined space, massive energy will emit by expanding gas.
o If you can create a cycle of this hundreds of times a minute, you have the core of a car engine.
§ Almost all cars use the four stroke combustion cycle
· Think of a potato gun. Now, replace the potato with a piston.
o The piston is connected to the crankshaft by a connecting rod
§ As the crankshaft revolves, it has the effect of “resetting the cannon”
o Intake stroke
§ The piston starts at the top, the intake valve opens, and the piston drops
· This allows the engine to take in a cylinder full of air and gasoline.
· This is the Intake stroke
· Only a tiny amount of gasoline is needed
o Compression stroke
§ The piston moves back up to compress the air/fuel
· Compression makes the explosion more powerful
o Combustion stroke
§ The spark plug emits a spark, lighting the gasoline
· The gasoline charge explodes, driving the piston down
o Exhaust stroke
§ Once the piston hits the bottom, the exhaust valve opens and the exhaust leaves the tailpipe.
· Now the engine is ready for its next cycle, so it repeats over and over again.
§ Parts of the engine
· Cylinder
o The core of the engine is the cylinder
o Pistons move up and down inside
o The above is a single cylinder, common in a lawn mower
o Most cars are 4, 6, or 8 cylinder
§ The higher the cylinder, the better performance
o Types of cylinder formation
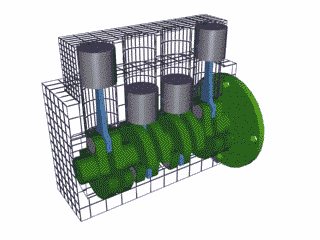 § L (line) formed in a line
§ L (line) formed in a line
§ V (angled) Shaped like a V
· Spark plug
o Provides the spark in the air/fuel compression
o Must spark at just the right time for proper function
· Valves
o Open and close for the intake stroke and for exhaust excretion
· Piston
o The grey shapes in the above pictures.
o Move up and down in the cylinder
· Piston rings
o Provides a sliding seal between the piston and cylinder.
o Provides two major purposes
§ Prevent excretion of the fuel/air mixture during compression and combustion strokes
§ Keep oil out of the space in the cylinder
· Connecting Rod
o Connects the piston to the crankshaft
o Can rotate at both ends so that piston can move and the crankshaft can rotate
· Crankshaft
o Works like a Jack-in-the-box, only the clown is the piston
· Sump
o Surrounds the crankshaft, containing oil
§ Oil used to lubricate and minimize temperature of the entire cycle
o Steam engines
§ External combustion engine
o Diesel
o Gas turbine engines
o Internal combustion engines
§ Gas is burned on the inside
§ The main idea/theory
· If you put a high power fuel (gasoline) in a small confined space, massive energy will emit by expanding gas.
o If you can create a cycle of this hundreds of times a minute, you have the core of a car engine.
§ Almost all cars use the four stroke combustion cycle
· Think of a potato gun. Now, replace the potato with a piston.
o The piston is connected to the crankshaft by a connecting rod
§ As the crankshaft revolves, it has the effect of “resetting the cannon”
o Intake stroke
§ The piston starts at the top, the intake valve opens, and the piston drops
· This allows the engine to take in a cylinder full of air and gasoline.
· This is the Intake stroke
· Only a tiny amount of gasoline is needed
o Compression stroke
§ The piston moves back up to compress the air/fuel
· Compression makes the explosion more powerful
o Combustion stroke
§ The spark plug emits a spark, lighting the gasoline
· The gasoline charge explodes, driving the piston down
o Exhaust stroke
§ Once the piston hits the bottom, the exhaust valve opens and the exhaust leaves the tailpipe.
· Now the engine is ready for its next cycle, so it repeats over and over again.
§ Parts of the engine
· Cylinder
o The core of the engine is the cylinder
o Pistons move up and down inside
o The above is a single cylinder, common in a lawn mower
o Most cars are 4, 6, or 8 cylinder
§ The higher the cylinder, the better performance
o Types of cylinder formation

 § L (line) formed in a line
§ L (line) formed in a line§ V (angled) Shaped like a V
· Spark plug
o Provides the spark in the air/fuel compression
o Must spark at just the right time for proper function
· Valves
o Open and close for the intake stroke and for exhaust excretion
· Piston
o The grey shapes in the above pictures.
o Move up and down in the cylinder
· Piston rings
o Provides a sliding seal between the piston and cylinder.
o Provides two major purposes
§ Prevent excretion of the fuel/air mixture during compression and combustion strokes
§ Keep oil out of the space in the cylinder
· Connecting Rod
o Connects the piston to the crankshaft
o Can rotate at both ends so that piston can move and the crankshaft can rotate
· Crankshaft
o Works like a Jack-in-the-box, only the clown is the piston
· Sump
o Surrounds the crankshaft, containing oil
§ Oil used to lubricate and minimize temperature of the entire cycle
o Steam engines
§ External combustion engine
Saturday, March 3, 2012
Sodium Stearate
Resource: http://en.wikipedia.org/wiki/Sodium_stearate
What the heck is sodium stearate?? Well, it is obviously a chemical. It is the sodium salt of stearic acid. The appearance of this salt is a white solid. The melting point is around 240 to 255 C. It is soluble in water. The salt itself is made up of carbon, hydrogen, oxygen, and sodium. The molecular formula is specifically C18H35NaO2.
This article is good for basic finds of sodium stearate and it's uses. The real life applications of sodium stearate is from soaps to food additives. One of the most common uses of sodium stearate is in soap. THe salt has both hydrophili and hydrophobic parts. It provides a formation of micelles, it basically provides a lipophilic environment for hydrophobic compounds. It can also be used in the pharmaceutical industry as a surfactant to aid the sulubility of hydrophobic compounds in production of many mouth foams. One of the uses I found in looking into sodium stearate is in deodorant stick products. It takes it's role by cleaning off the oils on the body. Sodium stearate is a common ingredient in many cosmetic products and possibly even food.
Subscribe to:
Posts (Atom)